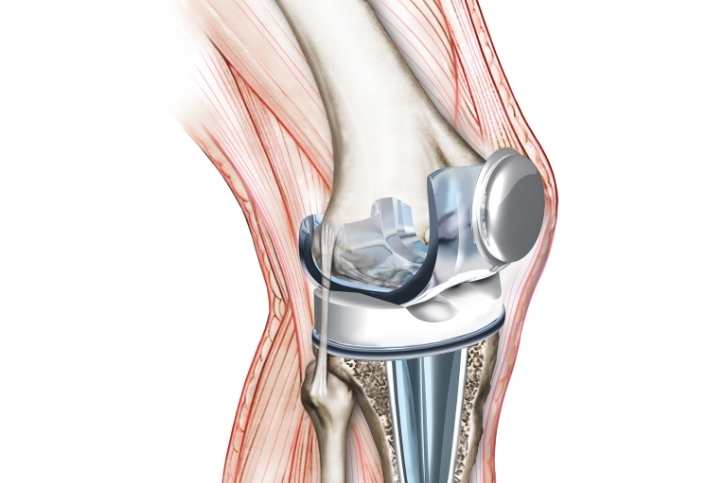
Exactech, a medical device business, is a frontrunner in producing implant devices for use in joint replacement procedures. There has been a recent massive recall of all Exactech knee replacement implant devices produced after 2004. A polyethylene insert component was found to have degraded due to a packing issue, prompting the recall of the implants.
There have been reports of Exactech knee implants failing unexpectedly (within a few months) due to inserts deteriorating, resulting in severe discomfort and prompting patients to seek revision surgery. If you have suffered from a knee replacement recall, we can help. File a claim to learn more.
In a product liability lawsuit, anyone who has had a knee replacement with an Exactech implant in the previous decade may sue the company for damages, including pain and suffering, medical costs, and lost wages.
Injured victims of defective Exactech knee implant systems, such as the Opetetrak® or Truliant® knee replacement systems, are the primary focus of Exactech attorneys.
After consulting with its clients, Exactech's legal team approached the JPML about initiating a new class action lawsuit. As a result, multidistrict litigation (MDL) is anticipated to be formed to house all federal recall claims. The court will hear the attorneys' consolidated arguments on September 29, 2022.
Undoubtedly, an Exactech class action lawsuit will be filed in multidistrict litigation. When a new class action lawsuit was filed to centralize all implant recall claims in federal courts, Exactech strongly backed it.
The Eastern District of New York was selected as the MDL's location at the request of both Exactech and the claimants. As a result, the JPML may create new multidistrict litigation (MDL) for the recall cases with Exactech's assistance.
Pretrial discovery will occur in New York City, whether you reside in California, Texas, Illinois, or another state. Your action will be tried in your home state if you and Exactech cannot settle during the discovery phase (and subsequent trials).
Less than a month ago, a motion to merge recall claims was brought to the JPML. Twenty-six cases involving the Exactech implant recall are now being litigated in American courts. Nine more cases related to the recall of Exactech's implants have been filed since then, increasing the total number of lawsuits related to the recall to 36. This suggests that the JPML will likely say yes to the request and set up a separate MDL for recall cases.
More implanted joint replacements from Exactech will be recalled in August of 2022. In a recent recall letter given out only last week, Exactech assured medical professionals that the recall of hip and ankle implants that began in February would not be prolonged. However, acetabular hip liners from Exactech's Connexion, Novation, and other implant systems are being called back.
Currently, the JPML is initiating new multidistrict litigation (MDL) class action complaint against Exactech.
Joint replacement surgical implants are Exactech's forte, and the firm exports its products worldwide (e.g., knee, hip, and ankle replacements). Regarding orthopedic surgery, Exactech is also a leading manufacturer of cutting-edge instruments and technology.
The Optetrak knee implant system was approved via the 501(k) FDA clearance procedure. That suggests it was never given a thorough examination. When first introduced in 1994, the Optetrak quickly found success among consumers. But unfortunately, many implants have been unsuccessful in recent years.
Patients whose Optetrak implants prematurely failed have filed knee replacement lawsuits against Exactech. The high failure rate of the first Optetrak device has been blamed on its design, namely the fact that it was "finned."
The company Exactech "quietly" abandoned the finned design in response. However, even after modifying Optetrak's design, the alarmingly high failure rates persisted. After investigating, Exactech found that the vacuum-seal packing was to blame for the premature degradation and failure of the polyethylene inserts.
Exactech began recalling all post-2004 knee replacements in 2021. Within the United States, the recall affects around 147,000 knee replacements. In total knee arthroplasty procedures, Exactech implant devices are often employed (TKA). TKA knee replacement surgery may help alleviate the discomfort of arthritis. In addition, total knee replacements are performed to treat knee injuries and conditions.
Multiple knee implant models manufactured by Exactech are covered in this recall.
Examples:
Exactech's knee implant devices were recalled due to a vacuum-seal packing failure. Unfortunately, the polyethylene component was damaged beyond repair due to excessive air exposure after shipping. In addition, the polyethylene implants were rendered less effective in the knee replacement system due to oxidation after shipping. Early failure of Exactech knee replacement systems was traced to polyethylene implants that had worn out due to excessive friction and wear.
Due to a design defect, the Exactech knee replacement system may fail sooner than expected. When knee implants fail, patients experience excruciating pain, swelling, and inability to walk. Replacement surgery is required when an implant fails.
A product liability lawsuit may exist for anybody with an Exactech knee replacement device implanted after 2004. For example, suppose you have an Exactech knee implant and file a complaint. In that case, you may be entitled to compensation for (a) your pain and suffering, (b) the expense of any necessary rehabilitation or revision surgery, and (c) lost wages.
If you have an Exactech implant in your knee or ankle, you should see a doctor. According to Exactech, doctors should maintain track of patients who received recalled implants and monitor them closely to detect any signs of implant failure.
Your Exactech knee or ankle implant may have been recalled if you received it after 2004. Exactech recalls all of its knee and ankle implants produced after 2004. All knee and ankle replacements manufactured by Exactech throughout the past decade are covered.
There is often confusion among joint replacement patients as to which manufacturer supplied their implant. Get in touch with your doctor or review your medical records if you underwent a total knee replacement and are curious as to whether or not Exactech manufactured your implant.
Exactech has contracted the services of Broadspire, a third-party administrator, to manage the claims process for individuals who have received recalled implants. If you submit a claim for reimbursement for the recalled implant, you may be waiving their ability to sue Exactech for damages. As a result, patients who submit claims for reimbursement to Exactech will get less money than they would if they took the company to court.
Exactech settlement projections are uncertain at this time. So, you shouldn't put any stock in the predicted settlement. However, compensation paid out in legal disputes and the Exactech recall of old serve as their basis. Reach out today to learn more about filing a claim.